
Medical innovation goes beyond obtaining RWD. It’s also about the full patient journey.
Often the key to accelerating drug approval or assess drug safety lies in connecting data silos – safely, reliably and compliantly.
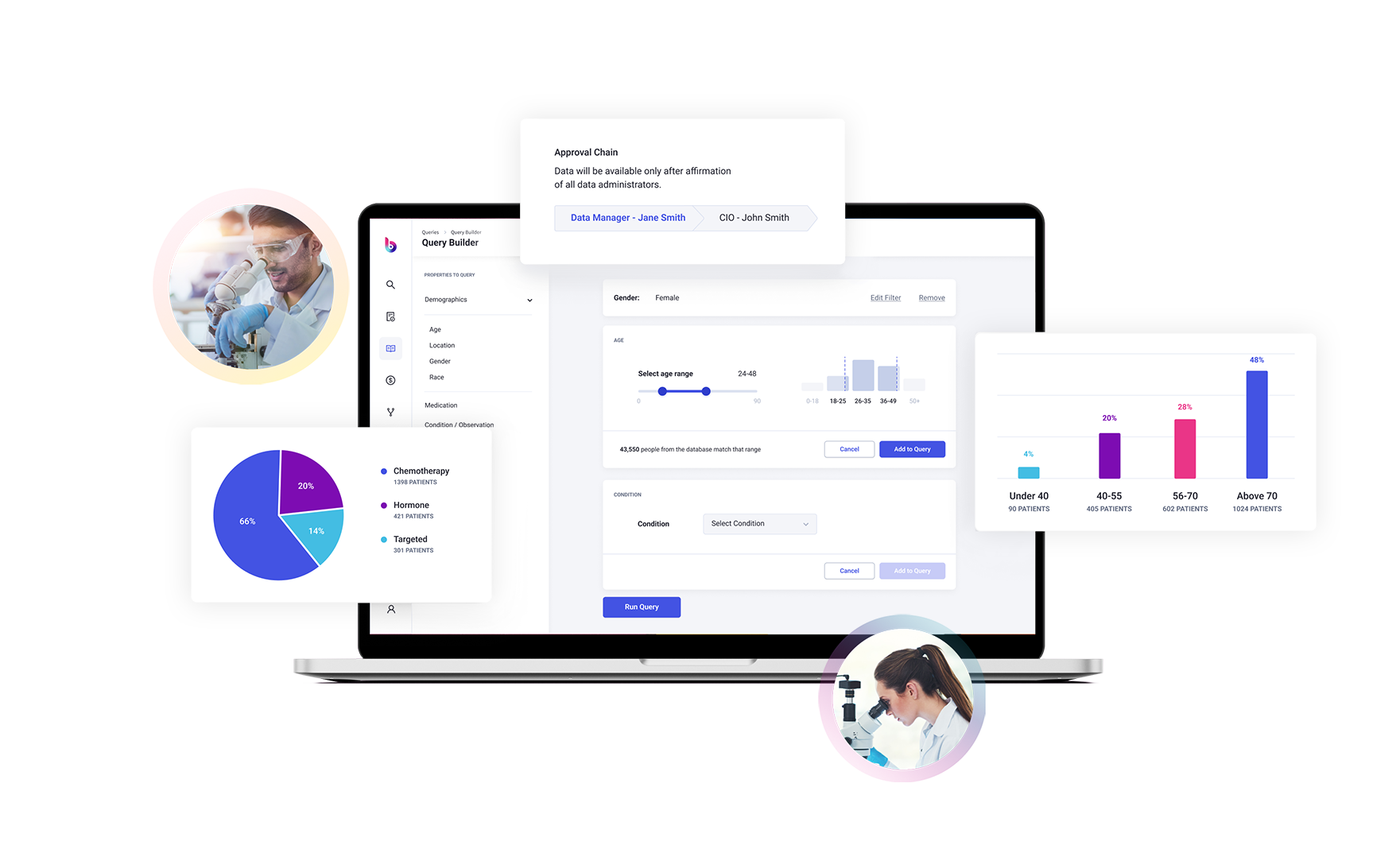
To this end, Briya brings game changing technology to the table.
Briya’s proprietary technology matches patient data across sources with unprecedented accuracy, while maintaining complete patient privacy, to bring you the most comprehensive longitudinal patient records available.


More accurate, relevant results provided with our expert AI curation.
Receive reliable patient-level data that has been standardized, de-duplicated, and flawlessly linked across multiple sources on demand, in real-time.

Smart contracts auto-enforce regulations such as HIPAA and GDPR as well as patient consent and DUA agreements.




2024 ©All Rights Reserved to Briya